Pharma Purity
Microbiological Testing for Pharmaceuticals
At Azecolab, we provide comprehensive microbiological testing services to ensure the safety, quality, and compliance of pharmaceutical products. Our state-of-the-art laboratory is equipped to detect and quantify microbial contaminants that can compromise product integrity and patient safety.
"*" indicates required fields
Why Microbiological Testing is Essential?
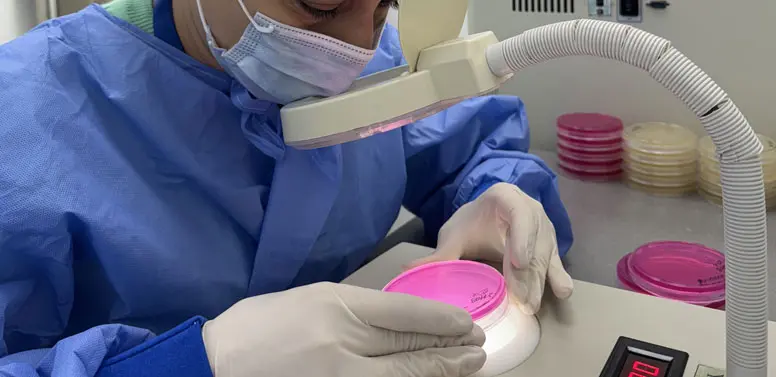
Microbial contamination in pharmaceuticals can lead to product recalls, regulatory non-compliance, and serious health risks. Our microbiological testing services help manufacturers:
Ensure product safety and efficacy
Maintain compliance with international pharmaceutical standards
Prevent microbial contamination in raw materials, formulations, and finished products
Our Microbiological Testing Services
Certain microorganisms must be completely absent from pharmaceutical products due to their potential to cause infections or degrade formulations. Our pathogen detection services include:
Escherichia coli (E. coli)
Indicator of fecal contamination and poor hygiene.
Pseudomonas aeruginosa
A waterborne bacterium that can contaminate aqueous pharmaceutical products.
Staphylococcus aureus
A common human pathogen that must be eliminated from pharmaceuticals.
Candida albicans
A fungal pathogen that can cause spoilage and infections.
Salmonella spp.
A highly pathogenic bacteria that should never be present in pharmaceutical formulations.
Fungal contamination can compromise product stability and safety. Our yeast and mold testing identifies and quantifies these microorganisms to prevent spoilage and ensure product quality.
We perform total aerobic microbial count (TAMC) testing to determine the overall bacterial load in pharmaceutical products. This ensures that the microbial population remains within acceptable limits.
For sterile pharmaceutical products, we conduct rigorous sterility testing to confirm the complete absence of viable microorganisms. This is critical for injectable drugs, ophthalmic solutions, and other sterile formulations.
Who Needs Microbiological Testing?
Pharmaceutical manufacturers ensuring batch release quality
Raw material suppliers verifying microbial purity
Contract manufacturing organizations meeting compliance standards
Research and development labs validating new formulations
Why Choose Azecolab?

Advanced Analysis
Cutting-edge laboratory technology for precise microbial identification and quantification.
Regulatory Compliance
Testing aligned with international pharmaceutical standards.

Expert Team
Experienced microbiologists and quality control specialists.
Fast Turnaround Time
Reliable results to support your production and compliance needs.
Download Our ISO/IEC 17025 Accreditation Scope
Accredited for Excellence in Testing & Calibration
Our laboratory is ISO/IEC 17025 accredited for both Testing and Calibration, ensuring the highest level of quality, reliability, and compliance in our services.

Testing Laboratory Accreditation – Verified for precision in environmental, chemical, and microbiological testing.

Calibration Laboratory Accreditation – Accredited for highly accurate instrument calibration and metrological traceability.
For any inquiries or custom testing/calibration needs:
Frequently Asked Questions
Why is microbiological testing necessary for pharmaceuticals?
Microbiological testing ensures that pharmaceutical products are free from harmful bacteria, yeast, molds, and pathogens. It helps maintain product quality, prevent health risks, and comply with regulatory standards.
How often should pharmaceutical products be tested for microbial contamination?
Testing frequency depends on regulatory requirements and manufacturing practices. Raw materials, in-process samples, and final products should be tested routinely, especially for sterile pharmaceuticals.
What happens if a pharmaceutical product fails microbiological testing?
If a product fails microbial testing, an investigation is conducted to determine the source of contamination. Corrective actions may include process adjustments, re-sanitization, and revalidation of manufacturing conditions.
What types of pharmaceutical products require sterility testing?
Sterility testing is mandatory for injectable drugs, ophthalmic solutions, intravenous fluids, and other sterile pharmaceutical formulations to ensure they are free from any viable microorganisms.
Why Choose Us?
Certified & Experienced Experts
Fast & Accurate Testing
Compliance with Regulatory Standards
Customer-Centric Service
Request a Consultation
Your Safety, Our Priority – Fill out the form below, and our experts will get back to you as soon as possible to assist with your inquiry.
"*" indicates required fields